What Is Oxidized in a Galvanic Cell Apex
A galvanic cell produces electrical energy through the conversion of chemical energy whereas the electrolytic cell carries out the conversion of the electrical energycurrent supplied to it into chemical energy. The silver metal B.

Electrochemistry Why Is Mass Gained At The Cathode Chemistry Stack Exchange
10 What reactions do occur at the cathode and the anode during electrolysis of acidulated water.

. Multiply each half-reaction by the correct number in order to balance charges for the two half-reactions. What is oxidized in galvanic cell made with silver and nickel electrodes. The mechanical energy is transformed to electrical energy in a galvanic cell whereas the electrical energy is converted to chemical.
In a galvanic cell the component with lower standard reduction potential gets oxidized and that it is added to the anode compartment. The following rules work for reactions performed in acidic or in basic solution. The nickel metal C.
The Ni2Ni 12 cell will therefore take in these electrons and move right to left. The voltage of the cell is an empirically measured quantity so must always have a ve value. Add your answer and earn points.
A galvanic cell is a chemical reaction that produces an electrical current and redox reactions are what. So to calculate E cell subtract the least ve E from the most ve E value. Electrochemical cell Galvanic Cell Electrolytic cell.
9 What happens when an electric current is passed through electrolyte. 1 point Mg - Mg 2 2 e- 2 Au 2 e- - 2 Au. The electrons are supplied by the species getting oxidized.
What is oxidized in a galvanic cell made with silver and nickel electrodes. The external battery supplies the electrons. A galvanic cell generates current and an electrolytic cell uses electrical energy to cause redox reactions to occur.
Separate oxidation and reduction half-reactions. The nickel ions D. Balance all atoms except for hydrogen and oxygen in each half-reaction.
The silver ions 1 See answer Advertisement Advertisement zachjames2005 is waiting for your help. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur which allow the continuous flow of electrons through the conductor while redox reactions are induced by an external source of current in an electrolytic cell. What is the total reduction potential of a cell in which Lithium Li is reduced and mercury Hg is oxidized.
Mg - Mg2 2 e- 2 Au 2 e- - 2 Au. 7 Is electrolytic cell and electrochemical cell same. Mg - mg2 2 e- Reduction.
Keep the vowels and consonants together AO CR III. But reduction and oxidation are always at the cathode and anode. -390 V What is true of electrolytic cells but is not true of galvanic cells.
They enter through the cathode and come out. 8 What energy conversion takes place in a nuclear submarine. Au e - - Au.
Galvanic cells always have positive potential. Galvanic cells also known as voltaic cells are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energyIn writing the equations it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations. To aid in this task a set of rules called the Half-Reaction Method has been devised.
E cell 08 025 105V. The second is therefore forms the cathode compartment. Galvanic cells also known as voltaic cells are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energyIn writing the equations it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations.
1 point A galvanic cell is a chemical reaction that produces an electrical current and redox reactions are what transpires in galvanic cells. They move from anode to the cathode in the external circuit. In this example they are already balanced.
Since almuninum has the lowest standard reduction potential xi-166V therefore it should be oxidized as follows. Give an example of a galvanic cell. Electrons do flow from the cathode to the anode in electrolytic cells which is backward from galvanic cells.
What kind of reaction occurs in a galvanic cell.
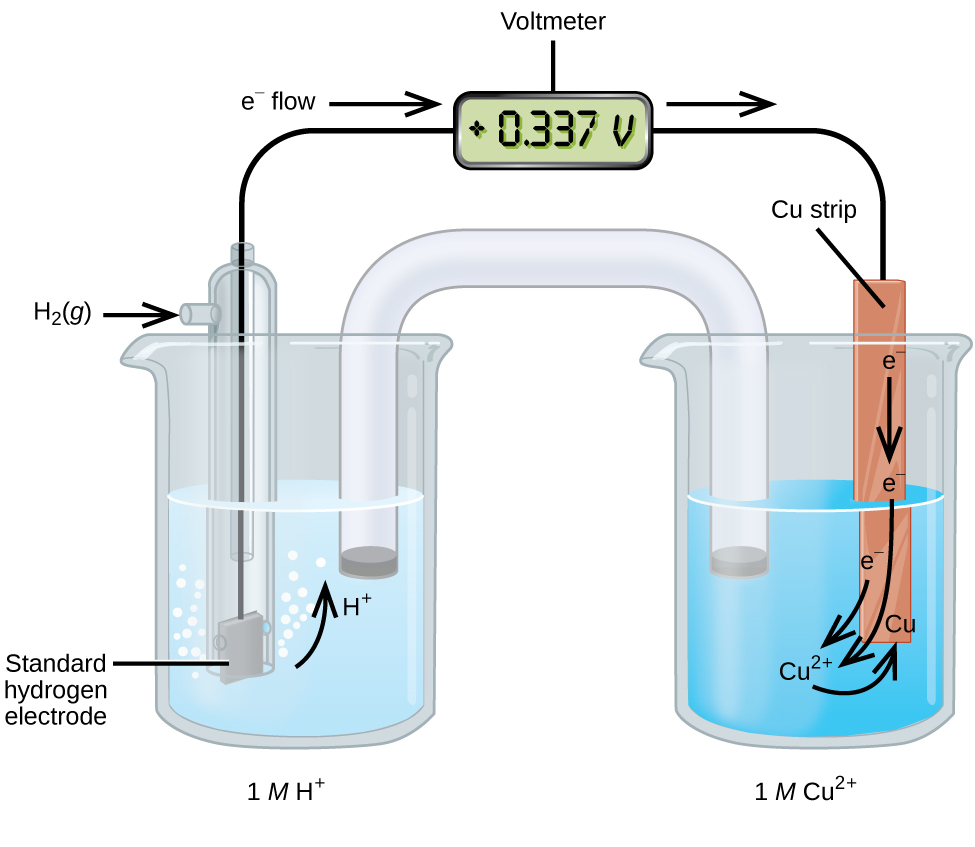
17 3 Standard Reduction Potentials Chemistry
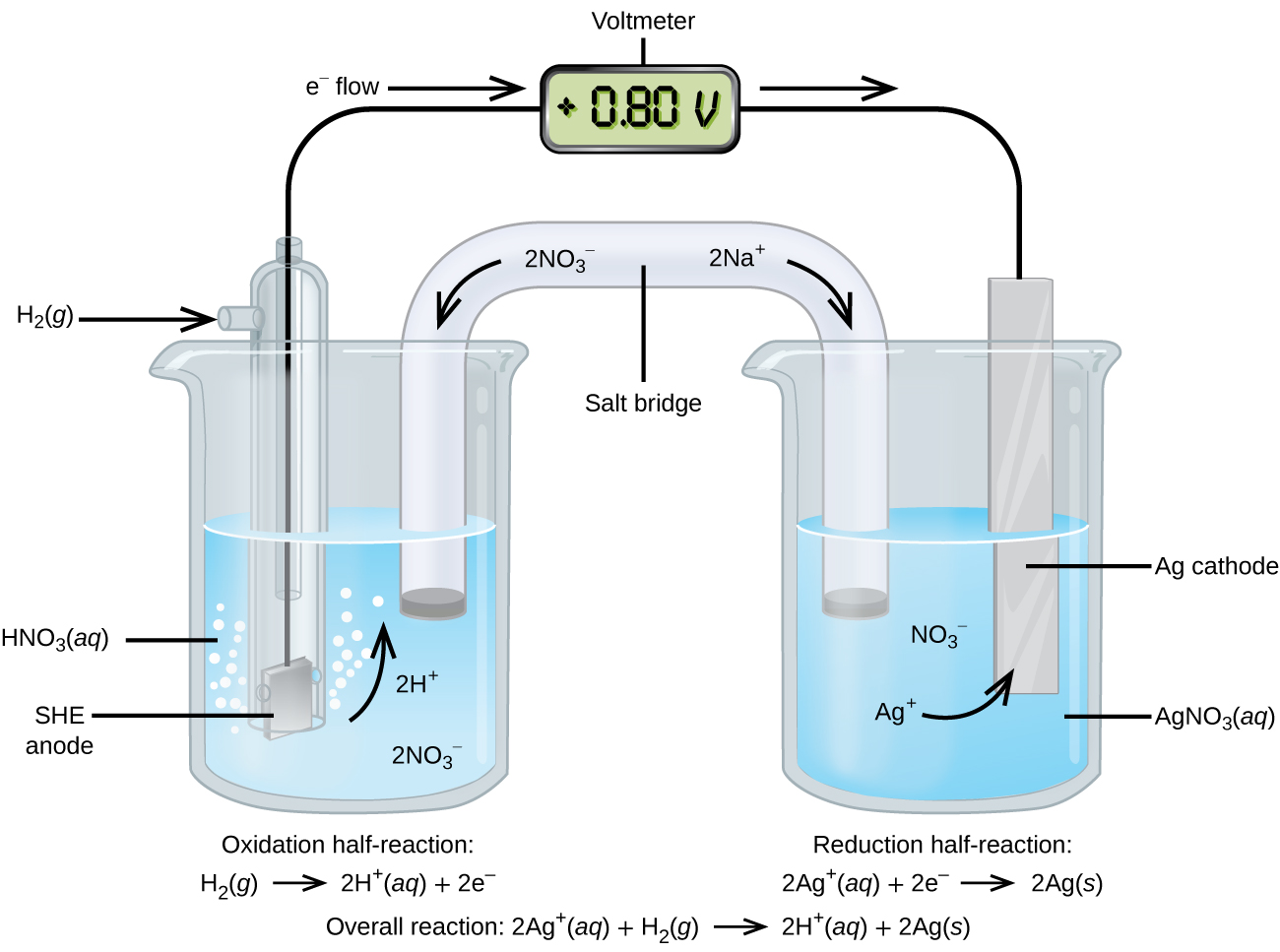
17 3 Standard Reduction Potentials Chemistry
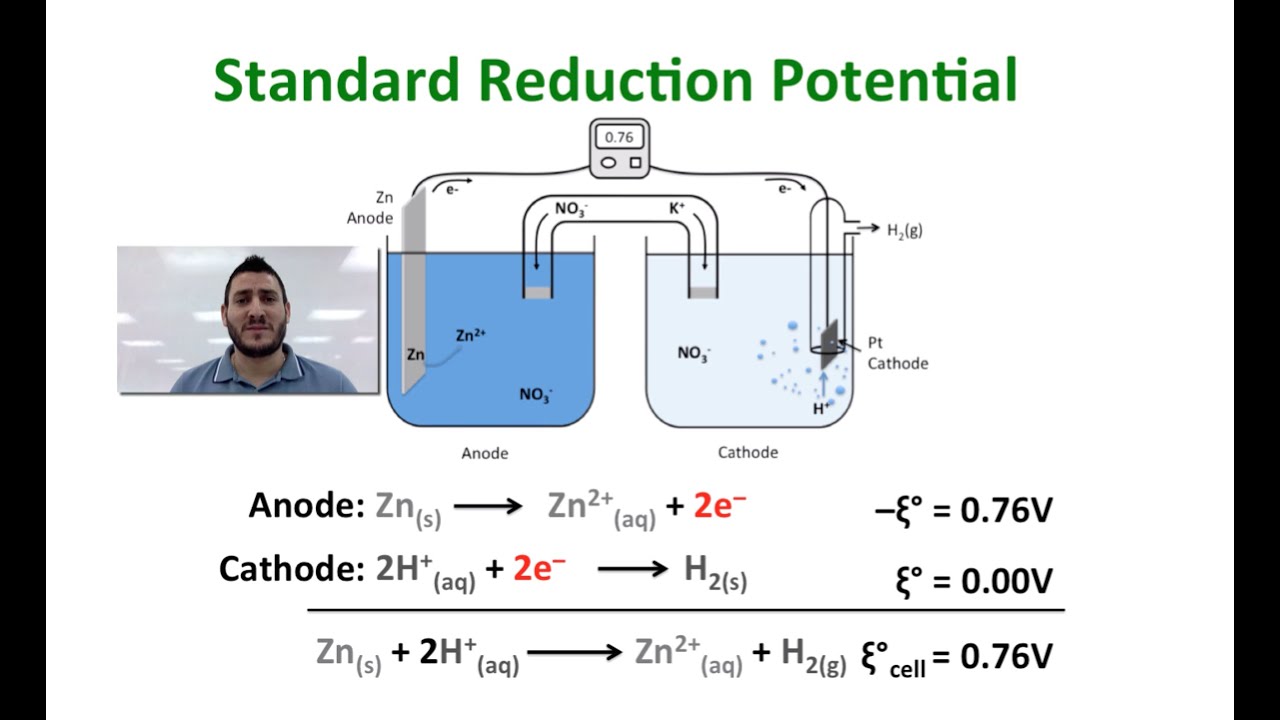
What Is The Voltage Of A Galvanic Cell Made With Zinc And Aluminum At Standard Conditions Socratic
What Is The Difference Between Electrolytic And Galvanic Cell Quora
Belum ada Komentar untuk "What Is Oxidized in a Galvanic Cell Apex"
Posting Komentar